Some people might it difficult to believe that Abilify can cause an addiction to gambling. In fact, one former Abilify consumer, who we will call Tony, developed such a compulsive gambling issue that his wife left him.
“I was on this medication for five years and my gambling addiction was so bad I lost my business and my wife,” says Tony. “Had I known that Abilify can cause gambling and sexual compulsions I would never have taken the drug. I can live with the weight gain but I’ll never get my wife back.” Tony stopped taking Abilify nearly a year ago. He no longer has a gambling addiction but he is now bankrupt and divorced.
Three major studies concluded that Abilify patients were able to control their gambling impulses when they discontinued the drug or had their dosage substantially reduced. The British Journal of Psychiatry in 2011 published a study that examined three Abilify patients who were addicted to gambling. Six months after taking it they were switched to another anti-depressant, and all of them no longer had a compulsion to gamble. A report in JAMA Internal Medicine (2014) found a substantial association between Abilify and gambling problems after studying the medical records of 1,580 patients reporting impulsive behavior issues. Also in 2014, the medical journal Addictive Behaviors published a study that found the same results.
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog



 Plaintiffs’ lawyers have requested a new multidistrict litigation for lawsuits alleging that Sanofi SA’s chemotherapy drug Taxotere can cause permanent hair loss in women, particularly those being treated for breast cancer.
Plaintiffs’ lawyers have requested a new multidistrict litigation for lawsuits alleging that Sanofi SA’s chemotherapy drug Taxotere can cause permanent hair loss in women, particularly those being treated for breast cancer.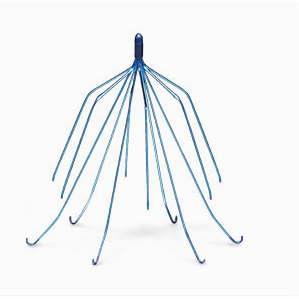 A device known as a vena cava filter (IVC filter) was implanted by doctors in hundreds of patients who are unable to take anticoagulants (blood thinners) in order to prevent blood clots from moving to the lungs. But recently two IVC filter devices named Cordis OptEase and Cordis TrapEase have came under scrutiny after lawsuits reveal potential design defects.
A device known as a vena cava filter (IVC filter) was implanted by doctors in hundreds of patients who are unable to take anticoagulants (blood thinners) in order to prevent blood clots from moving to the lungs. But recently two IVC filter devices named Cordis OptEase and Cordis TrapEase have came under scrutiny after lawsuits reveal potential design defects. Transvaginal mesh injuries can change a women’s life and cause a lot of pain. They often require multiple medical procedures or surgeries. Even then, medical professionals make no guarantee. Recent jury verdicts and settlements against the device makers have sought to assist women in dealing with the high costs of a mesh injury.
Transvaginal mesh injuries can change a women’s life and cause a lot of pain. They often require multiple medical procedures or surgeries. Even then, medical professionals make no guarantee. Recent jury verdicts and settlements against the device makers have sought to assist women in dealing with the high costs of a mesh injury.