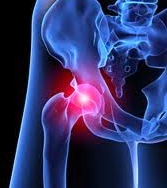
DePuy ASR metal on metal hip implant recipients in states with a 2 year Statute of Limitations, may lose their rights to file a lawsuit if they do not file their lawsuit before August 23, 2012. J&J’s DePuy unit recalled its 93,000 ASR hips worldwide in 2010, including 37,000 in the U.S., saying more than 12 percent of the devices failed within five years. Over 6,000 lawsuits in federal and state courts describe patients in pain and immobilized by joint dislocations, infections and bone fractures.
What is Statute of Limitations?
Every state has a law called the statute of limitations (SOL) that sets a limited time period in which a lawsuit can be filed. The reason for this law is that the courts want to have a finite time for folks to bring claims and not have an unending litigation time period. If a person with a legal claim fails to have a lawsuit filed within this time period, then their claim can be forever lost.
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog