http://www.youtube.com/watch?v=Z8YkrwxghUo&feature=player_detailpage
Johnson & Johnson Settlement Details
Dr Shezad Malik Law Firm has offices based in Fort Worth and Dallas and represents people who have suffered catastrophic and serious personal injuries including wrongful death, caused by the negligence or recklessness of others. We specialize in Personal Injury trial litigation and focus our energy and efforts on those we represent.
http://www.youtube.com/watch?v=Z8YkrwxghUo&feature=player_detailpage
Johnson & Johnson Settlement Details
Jurors in state court in Philadelphia found that Janssen failed to adequately warn doctors for Haley Powell, of the risks of Topamax before she gave birth to a son with a cleft lip.
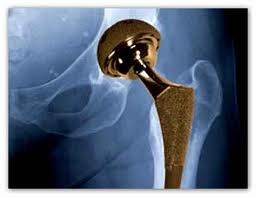 Smith & Nephew recently issued a safety notice that indicates the Birmingham hip failure rate appears to be higher than 1%, which exceeds the rate established for quality standards according to the United Kingdom’s National Institute for Health and Clinical Excellence.
Smith & Nephew recently issued a safety notice that indicates the Birmingham hip failure rate appears to be higher than 1%, which exceeds the rate established for quality standards according to the United Kingdom’s National Institute for Health and Clinical Excellence.
According to data collected since 2010 in the National Joint Registry of England and Wales, as well as the Australian Orthopaedic Associations National Joint Replacement Registry, the rate of Smith & Nephew Birmingham hip problems that were classified as a failure were 1.29% and 1.12%, respectively.
Johnson & Johnson Under Fire from Lawsuits
The settlement is the second multibillion-dollar settlement this month for J&J. The company, agreed November 4 to pay $2.2 billion to resolve criminal and civil probes into the marketing of Risperdal and other medicines.
http://www.youtube.com/watch?v=UrCdYKvJCl4&feature=player_detailpage
The proposed settlement is going to be presented in federal court and will need the blessing of the federal judge overseeing the DePuy ASR litigation, which at last count has passed the 12,000 mark for folks afflicted by the allegedly faulty hip implant.
http://www.youtube.com/watch?v=9XqN0hVm-Qg&feature=player_detailpage
Settlement Impacts Thousands of Patients
Robin Arnold and her husband, Felix, filed her case in the U.S. District Court for the Western District of Texas. They filed claims against Bard for negligence, failure to warn, design defects, manufacturing defects, breach of implied warranty, negligent misrepresentation and loss of consortium.
According to a Maryland jury, Takeda Pharmaceutical Co. failed to properly warn about the risks of its Actos diabetes drug and ordered the company to pay more than $1.7 million in damages. This was the second state court Actos bladder case injury case to go to trial. The judge overseeing the case immediately threw out the verdict.
Bayer 3rd Quarter Report
More than 300 Lawsuits in Federal MDL