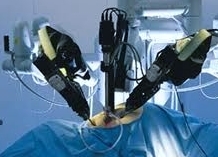
According to the jury in a recent Washingston state trial, Intuitive Surgical Inc., the manufacturer of the da Vinci Robotic surgical device systems wasn’t negligent in a wrongful death claim. The family of the plaintiff filed a lawsuit alleging that the company was negligent in its training of a doctor who performed the robot-assisted surgery on a patient who died.
Jurors in Port Orchard, Washington, reached their verdict after a five-week trial. The case was the first to go to trial of at least 26 lawsuits against Intuitive alleging injuries from its da Vinci robotic system, which was used in more than 300,000 U.S. operations last year.
Da Vinci Robot Cash Cow
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog