As a Texas Medical Doctor and DePuy ASR Failure Attorney, I am providing this update and commentary on a recent British database study.
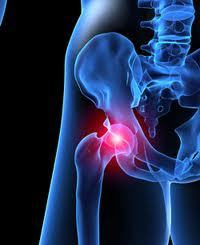
According to a British database, the National Joint Registry for England and Wales, which tracks hip replacement problems, a number of recalled DePuy ASR metal hip implants are failing within six years. Data from the National Joint Registry for England and Wales indicates that 29% of patients who received the DePuy metal-on-metal hip replacement have reported that they failed after only six years of use.
DePuy Orthopaedics, a division of Johnson & Johnson, issued a DePuy ASR hip recall last year, indicating that about 12% to 13% of these hips mail fail within five years. More than 90,000 DePuy ASR XL Acetabular Systems and DePuy ASR Hip Resurfacing Systems were sold worldwide before the metal-on-metal hip implants were recalled in August 2010. About 40,000 of those were sold in the United States.
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog