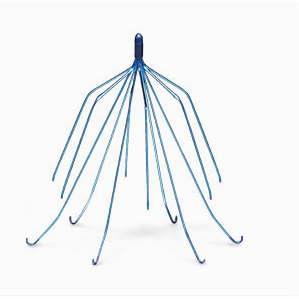
 A device known as a vena cava filter (IVC) was implanted by doctors in hundreds of patients who are unable to take anticoagulants (blood thinners) in order to prevent blood clots from moving to the lungs. Three devices manufactured by C.R. Bard quickly came under criticism after reports surfaced of complications.
A device known as a vena cava filter (IVC) was implanted by doctors in hundreds of patients who are unable to take anticoagulants (blood thinners) in order to prevent blood clots from moving to the lungs. Three devices manufactured by C.R. Bard quickly came under criticism after reports surfaced of complications.
C. R. Bard IVC filters catch the clots in the blood stream and, over time, the clots dissipate. The IVC filter is retrievable and it is not designed to be permanent.
Soon after IVC filters were widely used, the U.S. Food & Drug Administration (FDA) received hundreds of adverse reports about the retrievable filters. Reported complications included punctured organs, blood vessels and filter migration to different parts of the body.
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog



 While some in the medical community continue to debate whether or not Talcum Powder, also known as Baby Powder, may cause ovarian cancer, two recent lawsuits suggest it does cause ovarian cancer.
While some in the medical community continue to debate whether or not Talcum Powder, also known as Baby Powder, may cause ovarian cancer, two recent lawsuits suggest it does cause ovarian cancer. A 65-year-old Beaverton man who is dying of asbestos-related cancer – four decades after he was exposed to the material – was awarded $8.75 million by an Oregon jury.
A 65-year-old Beaverton man who is dying of asbestos-related cancer – four decades after he was exposed to the material – was awarded $8.75 million by an Oregon jury. The Food and Drug Administration (FDA) issued a drug safety communication stating that it has revised the warning labels of SGLT2 inhibitors, including Invokana, to include information about acute kidney injury.
The Food and Drug Administration (FDA) issued a drug safety communication stating that it has revised the warning labels of SGLT2 inhibitors, including Invokana, to include information about acute kidney injury. The popular Uber transportation services have raised many legal questions around the country, including who is responsible when a negligent Uber driver causes an accident.
The popular Uber transportation services have raised many legal questions around the country, including who is responsible when a negligent Uber driver causes an accident.