Stryker Rejuvenate cases, are heading to early trial dates, known as “bellwether” trials, which helps the parties determine how juries may respond to trial evidence and testimony.
Dr Shezad Malik Law Firm has offices based in Fort Worth and Dallas and represents people who have suffered catastrophic and serious personal injuries including wrongful death, caused by the negligence or recklessness of others. We specialize in Personal Injury trial litigation and focus our energy and efforts on those we represent.
Articles Tagged with Metallosis
Biomet Announces Global Settlement for Metal on Metal Hip Cases
Biomet Orthopedics the manufacturer of a line of metal on metal hips, announced a global settlement late yesterday, involving the hundreds of defective hip claims.
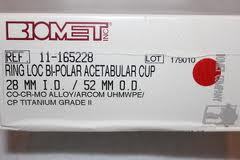 The agreement settles Biomet M2a Magnum hip lawsuits brought in the federal court system. Biomet agreed to pay $56 million to resolve lawsuits alleging that design problems with the metal-on-metal implant caused patients to undergo hip revision surgery.
The agreement settles Biomet M2a Magnum hip lawsuits brought in the federal court system. Biomet agreed to pay $56 million to resolve lawsuits alleging that design problems with the metal-on-metal implant caused patients to undergo hip revision surgery.
1,000 Biomet Defective Hip Claims
Stryker Rejuvenate Metal Hip Implant Lawsuits Continue to Settle
1,200 Stryker Hip Lawsuits
DePuy ASR Settlement Criteria and Process Explained
http://www.youtube.com/watch?v=Z8YkrwxghUo&feature=player_detailpage
Johnson & Johnson Settlement Details
Johnson and Johnson DePuy ASR Finalized Settlement Explained
Johnson & Johnson Under Fire from Lawsuits
The settlement is the second multibillion-dollar settlement this month for J&J. The company, agreed November 4 to pay $2.2 billion to resolve criminal and civil probes into the marketing of Risperdal and other medicines.
Dr Shezad Malik Explains Johnson and Johnson DePuy ASR Hip Implant Settlement
http://www.youtube.com/watch?v=UrCdYKvJCl4&feature=player_detailpage
The proposed settlement is going to be presented in federal court and will need the blessing of the federal judge overseeing the DePuy ASR litigation, which at last count has passed the 12,000 mark for folks afflicted by the allegedly faulty hip implant.
$4 Billion J&J DePuy Settles ASR Metal Hip Lawsuits
http://www.youtube.com/watch?v=9XqN0hVm-Qg&feature=player_detailpage
Settlement Impacts Thousands of Patients
Stryker Rejuvenate Hip Implants Ordered to Mediation
Stryker Rejuvenate hip lawsuits are on a fast track in New Jersey state court, with a court mandated mediation program in place to try and reach a settlement.
More than 430 defective hip product liability Stryker Rejuvenate and ABG II hip stems lawsuits are centralized in the Superior Court for Bergen County, New Jersey before Judge Brian R. Martinotti.
California DePuy ASR Hip Implant Failure Trial Settled
According to court documents, Johnson & Johnson has agreed to settle a DePuy ASR hip replacement lawsuit, on the eve of trial. The settlement agreement remains confidential.
Johnson & Johnson subsidiary DePuy Orthopaedics has agreed to settle a DePuy ASR hip lawsuit, the first bellwether trial in a California state court consolidation of ASR cases.
This DePuy ASR case that was set to begin later in October in California state court. See my earlier reporting on secret settlement negotiations regarding the DePuy ASR implants. This is overall good news for plaintiffs that the company is beginning to take the failure allegations seriously.
J&J Will Stop Sales of Metal-on-Metal Hip Replacements
As a Texas Metal on Metal hip attorney and medical doctor I am providing this important update to the folks injured by a defective metal hip implant. According to Johnson & Johnson (JNJ), it will stop selling metal-on-metal and ceramic-on-metal hip replacements because of a lower demand for the devices.This decision announcement was made in May 2013.
Metal liners in the Ultamet Metal-on-Metal Articulation and the Complete Ceramic-on-Metal Acetabular Hip System will no longer be available worldwide after August 31. J&J’s DePuy Orthopaedics unit will stop selling related products through 2014 to simplify and streamline its offerings.
Metal on Metal Hip Replacement Sales
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog

