Cook Medical Wins IVC Filter trial. Last week after a three-week trial, a federal jury found for Cook Medical, the maker of Cook Celect, and Gunther Tulip IVC (Inferior Vena Cava) filters.
Cook Medical Wins IVC Filter trial. Last week after a three-week trial, a federal jury found for Cook Medical, the maker of Cook Celect, and Gunther Tulip IVC (Inferior Vena Cava) filters.
Cook IVC Celect and Gunther Tulip Injury lawsuits
The jury found no liability in the first bellwether trial denying the claims of a Florida woman who alleged that the device caused her internal injuries.
The Cook IVC Filter injury case was tried in the U.S. District Court for the Southern District of Indiana and the jury found that there was no defectively designed device, this was the only claim heard from the plaintiff Elizabeth Hill’s initial complaint.
According to Hill, she had a temporary retrievable filter known as the Celect Cava implanted in 2010. Shortly after the implantation, Hill tried to have the filter removed. All initial attempts to remove the filter were unsuccessful, and upon further investigation, it was discovered that the filter had pierced a large blood vessel and small intestine, requiring surgery.
Cook IVC Filter Injury
Cook Celect and Gunther Tulip IVC filters are under fire over blood vessel perforation, vital organ damage and in severe cases catastrophic injuries requiring emergency surgery.
The Cook Gunther Tulip IVC Filter System was approved by the FDA in 2003. The Cook Celect IVC Filter System was approved by the FDA in 2008.
Cook IVC Filters approved by “fast track” FDA process
Cook Medical’s IVC filters were approved via the controversial FDA 510(k) fast-track approval process, which allowed the company to perform only minimal testing before selling the devices for use in patients nationwide.
FDA 510(k) fast-track approval process allows medical device manufacturers to sell devices with minimal testing “as long as they are substantially similar” to devices already approved under the more rigorous premarket approval process.
Cook IVC Filters Injury-FDA IVC Warnings
In May 2014, the FDA issued a safety alert in response to hundreds of reports involving IVC filters breaking inside patients and causing damage to the heart, lungs and other organs.
As a result, the federal regulators warned that retrievable IVC filters should be removed within about one to two months after the risk of a pulmonary embolism has passed.
Federal Cook IVC Filter trials set
All of the federally filed cases against Cook have been consolidated and centralized in the U.S. District Court in Indianapolis, as part of a multidistrict litigation or MDL. There about 3,000 injury claims filed against Cook in the federal system according to the latest court data from October. There are several bellwether trials scheduled in 2018.
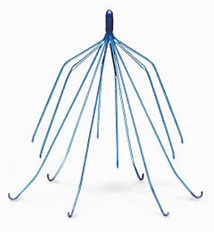
What are IVC Filters?
Inferior vena cava filters are cage-like devices inserted into inferior vena cava to prevent blood clots from the leg reaching the lungs, causing a pulmonary embolism, or blockage of an artery in the lungs. About 200,000 inferior vena cava filters are implanted nationwide each year.
There are also over 3,000 Bard Recovery filter and Bard G2 filter lawsuits pending in another federal MDL, which is centralized before U.S. District Judge David Campbell in Arizona.
IVC Filter Lawsuits
If you think you may have Cook Celect, Gunther Tulip IVC Filter implanted and have suffered IVC filter failure requiring emergency care, please call Dr. Shezad Malik Law Firm at 214-390-3189.
Read more here
https://www.dallasfortworthinjurylawyer.com/2017/06/cook-ivc-filters-injury.html
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog

