The Bard IVC Filter Injury lawsuits heading to trial set for 2017, according to the U.S. District Judge presiding over the coordinated pretrial proceedings in Phoenix, Arizona. Judge Campbell ruled that the first “bellwether” trial will probably go before a jury in fall of 2017. Since that date is a year away, more likely than not the date is liable to be moved as the cases get further developed.
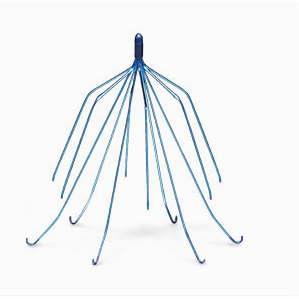
Recent medical studies have linked IVC filters to serious injuries and death in severe cases.
Federal Multidistrict Litigation
Lawsuits filed throughout the federal court system over severe and catastrophic complications with Bard inferior vena cava (IVC) filters have been centralized in the U.S. District Court for the District of Arizona, since August 2015.
There are over 1,000 Bard IVC Filter injury lawsuits and wrongful death claims before Judge Campbell. These Bard IVC Filter injury lawsuits have similar claims; that patients suffered serious injuries and on occasion died after receiving a Bard G2, Bard Recovery, Bard Denali, Bard Eclipse, Bard Meridian or other Bard IVC filter for prevention of a pulmonary embolism.
What is the problem with IVC Filters?
Recent medical studies have linked IVC filters to serious risks when the filter remains implanted in a person’s body for longer periods that what is recommended. Complications from prolong use of IVC filters have led to many hundreds of lawsuits recently across the United States.
Surgeons implant retrievable IVC filters in the veins of patients who are unable to take anticoagulants (blood thinners) in order to prevent blood clots from moving to the lungs. IVCs catch the clots in the blood stream and over time, the clots dissipate.
FDA IVC Filter Warnings
The U.S. Food & Drug Administration (FDA) received hundreds of adverse reports about the retrievable filters. Reported complications included punctured organs, blood vessels and filter migration to different parts of the body.
In 2010, the FDA warned the retrievable filters posed risks of filter fracture, device migration and organ perforation and should be removed as soon as the patient’s risk for blood clots subsided. The FDA updated safety communication in 2014, stating most devices should be removed between the 29th and 54th day after implantation. But the warning signs came too late for some.
IVC Filter Injury lawsuits on the rise
Plaintiffs began filing lawsuits across the country claiming the filters caused harm and death. At the center of these lawsuits are IVC filter manufacturers, C.R. Bard, Cook Medical and Cordis which according to estimates account for 75% of the IVC filter market.
Hundreds of similar Cook IVC filter and Cordis IVC filter lawsuits are also pending against these manufacturers. The IVC filters raising concerns in these lawsuits include:
- Cook Celect Filter
- Cook Gunther Tulip Filter
- Bard G2 Express Filter
- Bard G2 Filter
- Bard Recovery Filter
- Cordis Optease Filter
- Cordis Trapease Filter
The lawsuits allege that the manufacturers were negligent, failed to warn consumers, contained design and manufacturing defects, and that the manufacturers breached implied warranty.
Bellwether cases involving IVC filters manufactured by Cook Medical are also being prepared for trials, which are set to begin in October 2017.
Bard IVC Lawsuit: Heart Injury from IVC fragment
One of the first lawsuits was filed by Lisa Davis. She was implanted with a C.R. Bard G2 filter in 2006. The filter fractured in 2008 and migrated to her heart causing ongoing heart problems. Davis declined to have open heart surgery to remove the fragment and must take blood thinners for the rest of her life. She claims Bard failed to warn her physician of the G2’s risks and misrepresented the device as safe. She sued for physical trauma, anxiety and impaired ability to earn wages.
If you or a loved one developed complications after using an IVC filter, contact our office immediately. Our office handles IVC filters lawsuits from across the country.
 Dallas Fort Worth Injury Lawyer Blog
Dallas Fort Worth Injury Lawyer Blog

